Bad Drink, Good Blast…
Methanol is a toxic alcohol, capable of blinding and killing those who consume it… hence, a BAD drink!
The combustion of gaseous methanol results in the production of carbon dioxide, steam and fire! The expansion of the heated gases produces a characteristic “whoosh”… the GOOD blast!
2CH3OH(g) + 3O2(g) → 2CO2(g) + 4H2O(g)
Years ago, while performing a chemistry show to celebrate the opening of the 400 building, the exhaust from this demonstration managed to knock out a ceiling tile and completely destroy a separate demonstration!
Carbide Cannon…
Calcium carbide will react with water to produce acetylene gas…
CaC2(s) + 2H2O(l) → Ca(OH)2(s) + C2H2(g)
Acetylene gas forms explosive mixtures in air for pretty much all concentrations…
2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
And this is the basis for the carbide cannon!
Calcium carbide is mixed with water within a PVC tube, with the acetylene gas produced trapped by the placement of a small cup. A piezoelectric ignitor is then used to ignite the acetylene gas… producing a small bang and flash!
I used to perform this demonstration outside – of course, things change. A few years back I had the pleasure of seeing a half-dozen Campus Safety officers run around the building shortly after the ignition of the acetylene. They were less than pleased with the demonstration, and asked that I refrain from performing without prior notice…
Where’s the fun in that?
Combustible Bubbles…
Natural gas can be thought of as a mixture of combustible hydrocarbons and mercaptans (the smelly compounds!). As natural gas is primarily methane, we can write the chemical equation for this combustion process as…
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
Bubbles are produced upon flowing natural gas into a soap solution. Imagine scooping a handful of these combustible bubbles from a beaker… and then exposing the bubbles to a flame:

That’s right! You have a cool demonstration that results in the complete loss of hair on your hand…
Mighty Magnesium…
The combustion of magnesium is a sight to behold! The incredible temperature of the reaction (in excess of 3000 degree Celsius) results in a bright white flame…
2Mg(s) + O2(g) → 2MgO(s)
A fair amount of smoke (magnesium oxide) is also generated. I suppose this was the reason for the fire (smoke) alarm that resulted in an evacuation of two relatively large science complexes in my second year at Fullerton College.
You have to wonder how it was I kept my job!
oh sNap!
Ah yes, the reaction of sodium metal with water can be incredibly exciting!
As the sodium metal reacts with water, hydrogen gas is produced. The heat of the reaction ignites the hydrogen gas and, for a moment, the metal appears to be burning in water.
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
In actuality, the metal is melting as the hydrogen burns, eventually resulting in the production of enough hydrogen to generate a small explosion.
2H2(g) + O2(g) → 2H2O(g)
For those that are curious, the formation of a pink solution is due to the presence of phenolphthalein indicator. The basic conditions created by the formation of (sodium) hydroxide change the solution from colorless to pink… Good times, eh?
Thermite…
What can I write that could possibly add to the thrill of preparing molten iron? Right?
Here we have the reaction between iron(III) oxide and powdered aluminum. A piece of magnesium ribbon is used as a fuse… the burning magnesium initiates the reaction, which results in the release of significant amounts of energy (heat). The products of this reaction include molten iron, which falls from the terra cotta pot into a bucket of sand.
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s)
Before you make the mistake of thinking that this reaction is limited to the “classroom”, it was used to weld the railroad rails for the transcontinental railroad!
I should add that this reaction is still used to this day to weld railroad rails…

It’s amazing what you can be paid to do these days. Right?
Cellulose Nitrate…
Call it nitrocellulose, cellulose nitrate, flash cotton, or just guncotton… Me? I call it a good time! Cellulose nitrate was definitely on my bucket list for demonstrations since first witnessing it many years ago at the University of Utah… and, again, at CSU Fullerton. The production of cellulose nitrate is straightforward and should be performed by all chemists at some point in their career.
With sulfuric acid as a catalyst…
3HNO3 + C6H7(OH)3O2 → C6H7(ONO2)3O2 + 3H2O
Or…
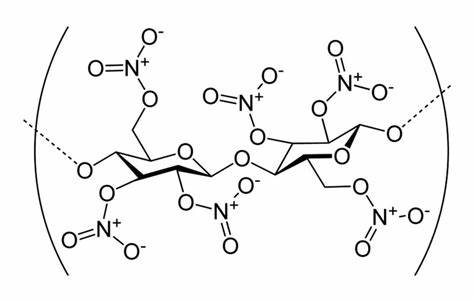
And upon exposure to heat… well, check out the video and see for yourself!
Silver Tree…
I could write that with a standard reduction potential of 0.80 V, the silver ion is a strong oxidizing agent… but it’d put you to sleep, right? Rather than dwell on the chemical properties of the silver ion, marvel at this relatively cool chemistry! A copper wire is first coiled around a pencil and then submerged within a solution of silver nitrate. Within a matter of minutes, the silver ions are reduced to form crystals of silver metal on the copper wire, which is oxidized to form a blue solution of copper(II)
2Ag+(aq) + Cu(s) → 2Ag(s) + Cu2+(aq)

If this isn’t cool enough… consider that I will filter the silver before disposing of the waste solution and with (many, many) years of collection, produce these fine looking bars of silver!

(For my eventual retirement…)





